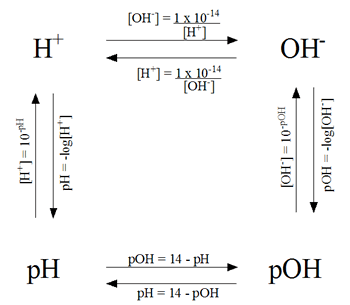
Last Friday we learned about how to find the specific pH of an acid or a base. pH tells how acidic/basic a solution is. We found it by taking the hydrogen ion concentration and putting it into an equation.
The OH- stands for hydroxide ion, what is normally found in bases. It is possible to know the OH- concentration and finding the pH by using these formulas.
Above is a pH scale that gives a few examples of acidic and basic solutions. Solutions form 0-6.9 are acidic and solutions that are form 7.1 to 14 are basic. Pure water is a neutral solution, which is represented by 7 on the pH scale.
More info and practice:
Interactively shows pH:

No comments:
Post a Comment