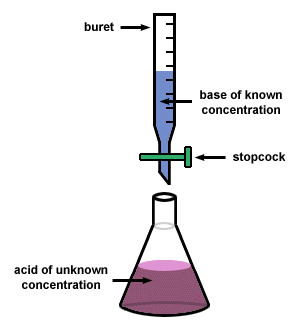
The last two labs we've had have been over titrating an acid with sodium nitrate, to find exactly how much acid is in the solution. Titration works by taking a an acid with an indicator (another solution) and putting a base into it. The base will react with the acid until there is no more acid to react to it, where the base will combine with the indicator, making a color change. The first lab we found how much acetic acid was in vinegar, then the second one was over an unknown acid. In both labs we found a standard (titrating KHP with NaOH3) and use the resulting numbers to find the molarity of the NaOH3, then use that number to calculate the amount of acid.
Tuesday, February 9, 2016
Titrations
The last two labs we've had have been over titrating an acid with sodium nitrate, to find exactly how much acid is in the solution. Titration works by taking a an acid with an indicator (another solution) and putting a base into it. The base will react with the acid until there is no more acid to react to it, where the base will combine with the indicator, making a color change. The first lab we found how much acetic acid was in vinegar, then the second one was over an unknown acid. In both labs we found a standard (titrating KHP with NaOH3) and use the resulting numbers to find the molarity of the NaOH3, then use that number to calculate the amount of acid.
Labels:
Acids and Bases
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment