Tuesday, May 10, 2016
Kinetic Theory
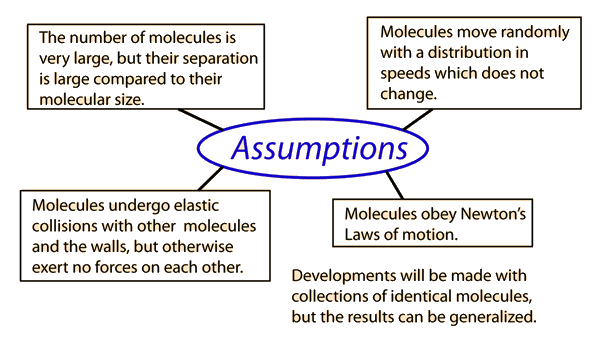
Kinetic theory of gases is the bases for all seven of the gas laws. In this theory, subatomic particles in a gas, either atoms or molecules, are in an excited state of motion and the particles themselves have a randomness to them. They bounce against the wall of the container they are in and against each other. This theory is used for macroscopic properties of gas (gas as a whole, not individual particles), such as temperature, pressure, and volume.


Labels:
Gas Laws
Subscribe to:
Post Comments (Atom)
Thanks for going over the Kinetic Theory of Motion, it really tripped me up during the quiz.
ReplyDeleteThis was a really good explanation of the concepts I remember studying and alot of the conceptual questions tripped me up hopefully this will help me for the test.
ReplyDelete