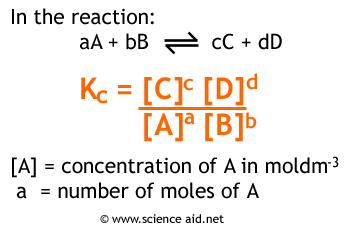Waves, we leaned about waves. And no, not the really cool ocean waves, but the waves of subatomic particles (electrons). So apparently electrons like to move in a wave like nature, where they oscillate back and forth. I don't think anyone knows why they do this, but rather accept it was reality. There is also many things that are to be known about these waves:
Using math, it becomes possible to find the values of some of these variables (wavelengh and amplitude) by using he equation: