Oxidation series tell how easily an element oxidizes compared to another element. Metals only compare with metals and non-metals with non-metals. Chemprime gives a brief explanation of oxidation series.
In redox reactions, the element losing electrons are oxidized and the element that gains electrons are reduced element. The oxidized elements is the reducing agent and the reduced elements is the oxidizing agent.
Honesty I find most of this stuff confusing and am going to have to study to have any clue what to do on the lab tomorrow. I don't remember anything from the last lecture.
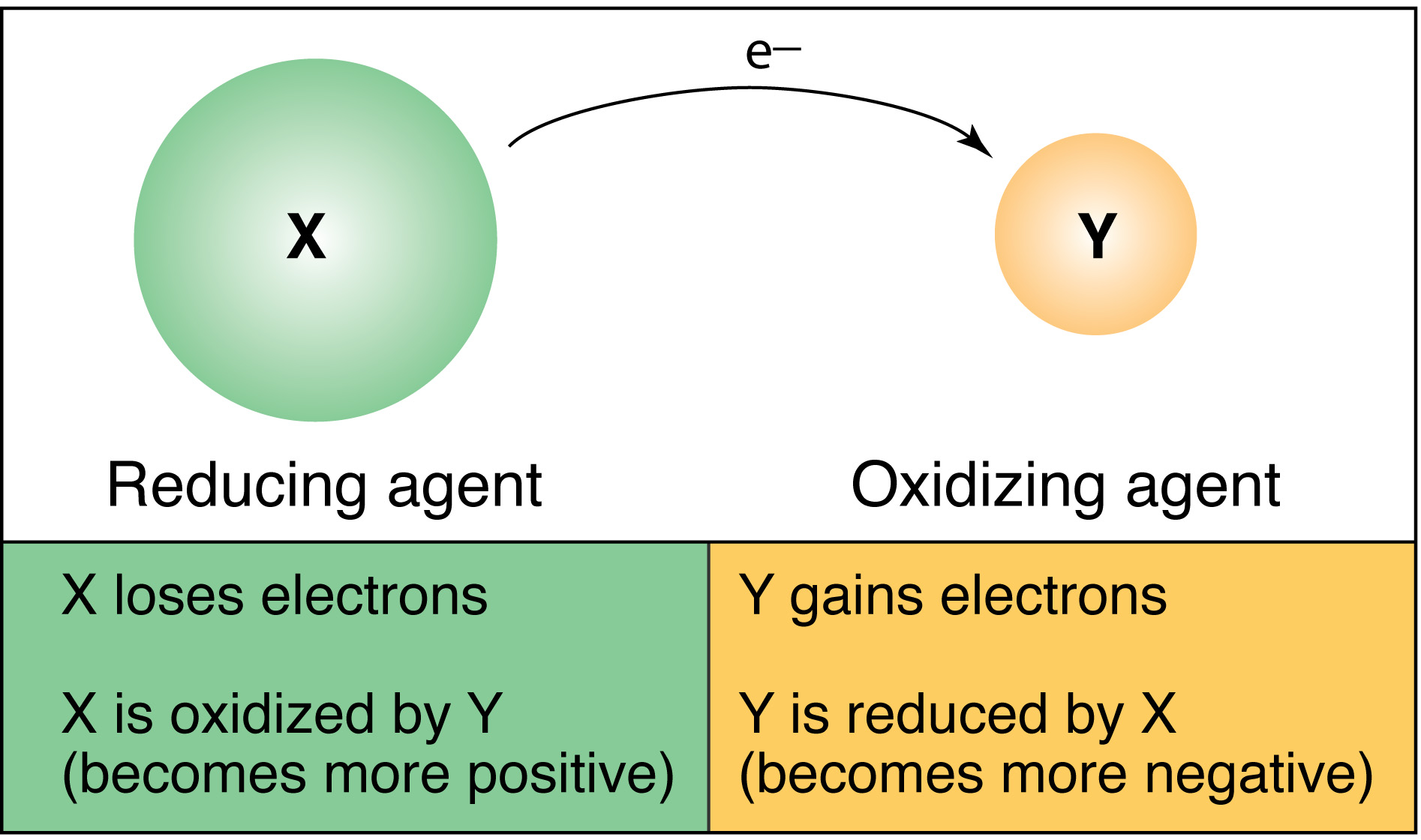
I wish I had seen this diagram before the test, it would have made life a lot easier
ReplyDelete