Here is a couple of was to study I found:
Tuesday, December 15, 2015
Thoughst on Unit Test
Overall I didn't think this test was especially hard; but, like this quiz, was time consuming. It was only twelve question long but took me about 45-50 minutes to complete. Many of the question involved multiple equation inside the problem, like for finding the percent yield of a certain element given the formula, which takes about three equations to find out. Fingers crossed that I did well, which I feel like I did.
Last Lab and Percent Yield
Last Thursday we started on a three day lab that involved a reaction between iron and copper (II) chloride. On Friday we took the iron (a nail) out of the solution and some pretty cool but kinda nasty stuff had happened. Some copper was left in the bottom of the jar while iron off of the nail had dissolved into the liquid. The jar was then dumped of solution, rinsed, and left to dry. On Monday when we came back, we measured out the amount of copper in the jar. Using these measurements (weight of nail before and after reaction, weight of copper, and weight of jar) I was able to find the percent yield of the reaction was around 75%. The percent yield is the actual amount divided by the theoretical amount. This shows us how there was other stuff going on inside the reaction that we have no control of. More info on percent yield here.
Wednesday, December 9, 2015
Limiting Reagent
The second lecture my class had on stoichiometry was to find which reactant in a compound was the limiting reagent. In layman's terms, the limiting reagent is the compound that is totally used up in a chemical reaction, all other compounds are the excess reactants.
I find that this picture really helps explain exactly what is going on. Basically it shows that no matter how many tires there are, if their are only eight car bodies, only 8 cars can be made. The 16 extra tires is the excess reactant. This website shows the math and what is going on and links to a few practice problems.
Monday, December 7, 2015
Stoichiometry
Stoichiometry is a section of chemistry that involves using relationships between reactants and/or products in a chemical reaction to determine desired quantitative data. Basically this means math. It can be easily visually in a flow chart, where the compound is placed into moles, converted, and the converted compound is changed back into mass, volume, or number or partials. In class we only did conversion involving mass. The result of the conversion told us how much of amount of a species can be used to create a product in a chemical reaction.

Confused? Here is a Crash Course video that can help.

Confused? Here is a Crash Course video that can help.
Tuesday, December 1, 2015
Metals Lab
Today we did a lab that involved taking six different metals and pouring chemicals on them to see of they react with each other, and it was pretty cool. Some of the metals started to bubble and smoke when they came into contact with each of the chemicals, and others dissolved into a puddle of goop. On of the metals that we used was calcium which turns out to be pretty reactive, and lead was not at all. Sadly I forgot to take a picture of the reactions at work but here what the one of calcium and water together looked like:
Monday, November 30, 2015
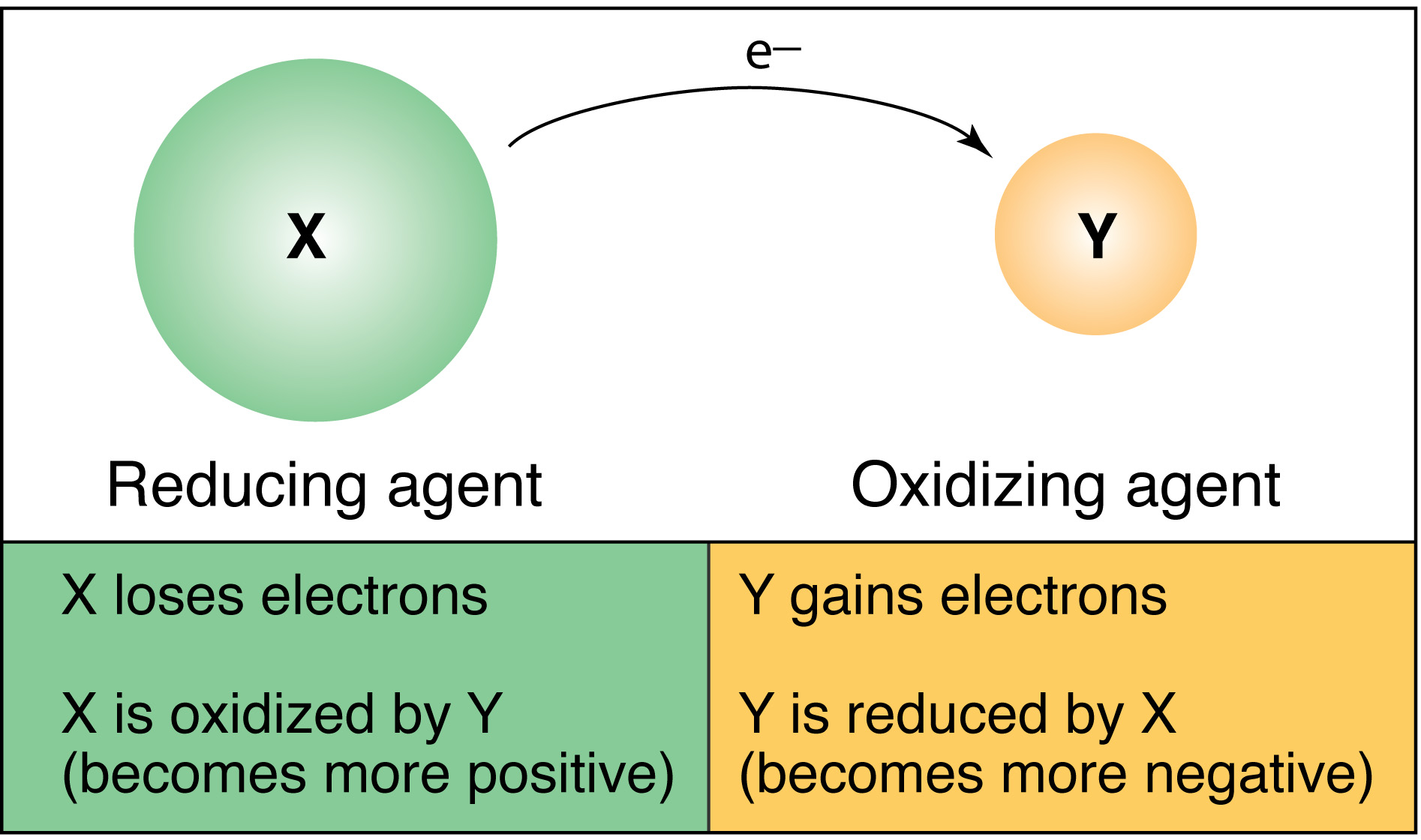
Redox: Transfer of Electons
In redox reactions, electrons are transferred from the metal to the non-metal. My class and I leaned that redox reactions are either single-replacement, synthesis, or decomposition and if a reactions occurs depends on the oxidation number and the oxidation series of the elements and compounds involved. More info on the oxidation rules can be found on chemwiki.
Oxidation series tell how easily an element oxidizes compared to another element. Metals only compare with metals and non-metals with non-metals. Chemprime gives a brief explanation of oxidation series.
In redox reactions, the element losing electrons are oxidized and the element that gains electrons are reduced element. The oxidized elements is the reducing agent and the reduced elements is the oxidizing agent.
Oxidation series tell how easily an element oxidizes compared to another element. Metals only compare with metals and non-metals with non-metals. Chemprime gives a brief explanation of oxidation series.
In redox reactions, the element losing electrons are oxidized and the element that gains electrons are reduced element. The oxidized elements is the reducing agent and the reduced elements is the oxidizing agent.
Honesty I find most of this stuff confusing and am going to have to study to have any clue what to do on the lab tomorrow. I don't remember anything from the last lecture.
Sunday, November 29, 2015
Acid-Base Reactions
Acid-Base reactions driving force is the productions of water. In additions, they produce a salt. Acid-Base reactions are chemical reactions that take an acid and a base to form water and a salt, which is the cations of the base and the anion of the acid. More info on acid-base reactions here.
Acid-Base reactions are similar to double replacement reactions, but differ in the fact that they only have one ionic compound while double replacement has two. Reactions also differ in the fact that weak acids and bases can effect the outcome differently than strong acid and bases. Basically strong acids and bases completely disassociate while weak acids and bases don't. More information on the specifics can be found here.
Monday, November 23, 2015
Chemical Reactions and Precipitation
Another long and hard unit where nomenclature is crucial to the success on the tests and quizzes. Chemical reactions is the topic of this unit, where I have to learn if and how two different chemicals join together in a chemical reaction. Reactions only occur if their is a driving force present in the products of the chemical equation. We leaned that chemical reactions either: form a solid, form water, transfer electrons, or form a gas. First we went over double replacement reactions, which form a solid from two aqueous solutions, and is one of the four basic chemical reactions. More information on them here.
 |
Sunday, November 22, 2015
Chemical Reactions Lab
This is our first lab of the chemical reactions unit and it was pretty cool. We mixed 10 drops of 9 different kinds of chemicals with each other and saw which ones reacted and which ones didn't. A little less than half changed colors, which means the two chemicals had a reaction with each other. All of the reactions where called precipitation reactions, when two soluble salts combined together to get one insoluble one. Below is a picture of one of the plates of mixed chemicals.

Tuesday, November 17, 2015
Post-Test Terrors
Oh my, hardest test ever. I've never seen a test where so many people in my class, way over the majority of the class, not finish, many of the people weren't even close. In hindsight I should have have studied a little bit more, especially my polyatomic ions. I ended up forgetting what the formula for bicarbonate was, and was unsure on a few others, probably should have done this a couple of times before hand. I also missed an easy question because I thought 6.02 x 10^23 was Mendell's constant and not Avogadro Number so that stinks. Ended up guessing on like 7 questions, but was happy she gave an free one (just told us which one to circle).
Friday, November 13, 2015
Upcoming Test
I hate Monday tests. Or at least extremely dislike. It means I have to study over the weekend or at least look over the materials and I'm not a huge fan of that, rather do that on the weekdays.
Over all I think I'm gonna gonna do pretty well, at least on the first half of the unit. I ended up getting 15 out of 17 correct on the test, or a 88%. I'm still kind of confused at the point of the both empirical and molecular formulas, and when I think upon it further, the latter of the two makes more sense. Why would there be a formula which isn't even the actual formula of the compound (empirical)? Found some more information upon both of these formulas here.
Thursday, November 12, 2015
Empirical and Molecular Formula
The empirical formula is the simplest formula for a compound. This is different form the molecular formula, which is based of the actual number of atoms in a compound. Now I don't really understand why there are two types of formulas if the both tell you basically the same thing. I would think that all formulas would be reduced down to their simplest form anyways.
This chart shows the difference, and sometime there's none. The way to find the empirical formula from just knowing the elements present and the percent abundance is kind of confusing but manageable. The math behind it can be found here.
Tuesday, November 10, 2015
Post Quiz Thoughts
Just had the weekly quiz today, and oh my. It really wasn't that terrible, but the first question made me think my teacher had it in for me. It was one of those multiple step problems and in hindsight I did it totally wrong. Turns out I should leaned about converting moles into volume of gasses, as that question pertained to chlorine gas.
After the first page, which too a long time, the rest of the quiz wasn't so bad. I answered all but one other question confidently, and that was because I was totally clueless of the chemical formula of the substance mentioned in the question.
Had a lab on hydrates yesterday, and it was pretty interesting. If you want to know more about them look here. The lab was fun because I got to use a Bunsen burner for the first time, and that weird looking contraption used for starting the flame is pretty cool too. Basically we just heated the water out of a hydrate then figured out how water was in the chemical formula.
After the first page, which too a long time, the rest of the quiz wasn't so bad. I answered all but one other question confidently, and that was because I was totally clueless of the chemical formula of the substance mentioned in the question.
Had a lab on hydrates yesterday, and it was pretty interesting. If you want to know more about them look here. The lab was fun because I got to use a Bunsen burner for the first time, and that weird looking contraption used for starting the flame is pretty cool too. Basically we just heated the water out of a hydrate then figured out how water was in the chemical formula.
Sunday, November 8, 2015
Moles and Measurement
Back to moles, the mathematical kind. Now I kinda understand how their used and what for. One mole stands for 6.02 × 10^23 representative particles. Essentially representative particles is the estimated number of particles in an sample.
Moles are used for converting the mass of an sample into the number of atoms it contains and vise versa. It kinda works like a middle man between measurements. To do these conversions, you also need to know atomic mass, which is found on the periodic table. A mole map is helpful in these conversions.
Still confused? This website should help.
Thursday, October 29, 2015
Unit Conversion Project
This unit's project, the second one that is, is converting measurements of food in recipes from standard to metric (SI) units. This project was not really that confusing, and is very relevant to our unit, Matter and Measurements. A useful website I found for turning food measuring units (cups, teaspoons, tablespoons) into grams and liter.
For some of the recipes that I used one of my mothers cookbooks, Better Homes and Gardens. I found the website here.
For some of the recipes that I used one of my mothers cookbooks, Better Homes and Gardens. I found the website here.
Tuesday, October 27, 2015
Dimensional Analysis
Dimensional analysis is basically converting measurements into different units.While I have learned this before in some of my other classes, Mrs. Frankenburg taught it in such a way that it made more sense then some of my other teachers. Sadly I have to remember a lot of the conversions, so that could trip me up on the test on Thursday. I found a website that has most, if not all of the conversion on it.
We also learned about Kelvin today, the temperature that it. Kelvin measures the kinetic energy of particles in a sample. Kelvin was created the Kelvin scale with number of absolute zero (no heat in sample) being equal to zero. Creating a new scale wasn't the only thing that he achieved as seen here.
We also learned about Kelvin today, the temperature that it. Kelvin measures the kinetic energy of particles in a sample. Kelvin was created the Kelvin scale with number of absolute zero (no heat in sample) being equal to zero. Creating a new scale wasn't the only thing that he achieved as seen here.
Friday, October 23, 2015
Moles and Chemisty
A mole is equal to 6.0221415×1023, that is, in the SI unit of measurement. Chances are you know of moles as little mammals that dig underground, and like in my house, wage war on the front lawn, looking something like this.
Due to a national mole day (yes it's a real thing, there is even a National Mole Day Foundation), my classmates and I made a stuffed animal mole, and Mrs. Frankenburg loved them. While I don't see any real connection to stuffed animals and chemistry, I did get a day off of lecture so I'm not complaining.
Subscribe to:
Posts (Atom)